
Multiple sclerosis (MS) may one day be cured with brain stem cells injections, as a groundbreaking experiment by Cambridge researchers demonstrated that the illness was slowed down by the injections.
This is the first time the technique has been used to human subjects; a total of 15 individuals with substantial symptoms and secondary MS were included in the trial.
The approach was shown safe and effective in animal studies, and the first phase of human clinical trials was likewise successful.
One year after therapy, none of the subjects had worsened, and there were no serious adverse effects.
Neural stem cells, a kind of blank canvas that may grow into whatever tissue required, are taken from a donor and injected deep into the brains of MS patients.
With a skull cannula, patients get injections of stem cells that were grown in a lab from the brain of an aborted baby.
Also Read : Cancer Cells Modify Cell Competition to Boost Their Own Capability to Invade
The stem cells given to all 15 patients were identical clones derived from a single donor.
The cells were extracted, stored, frozen, and cultured in a laboratory. Additionally, one donor supplied stem cells for an ALS research study (motor neurone disease), which is being conducted by the same Cambridge team that conducted the MS experiment.
Larger phase two studies are now being initiated as a result of the MS and ALS trials’ remarkable results.
Before the end of the year, around 40 patients with fast-progressing ALS—a degenerative illness that advances similarly to MS but more aggressively—are anticipated to be recruited.
Phase two clinical trial approved
The Italian regulatory agency, AIFA, has also approved a phase two clinical study for MS, as far as The Telegraph is aware.
Co-author of the research and University of Cambridge professor of regenerative neuroimmunology Prof. Stefano Pluchino predicted that stem cell treatment will one day treat both MS and ALS.
Although there are medications that lessen the intensity of symptoms for the more than two million MS patients worldwide, two-thirds of them develop a crippling secondary phase within 30 years.
The illness arises from a compromised immune system, leading white blood cells to target brain tissues rather than outside invaders.
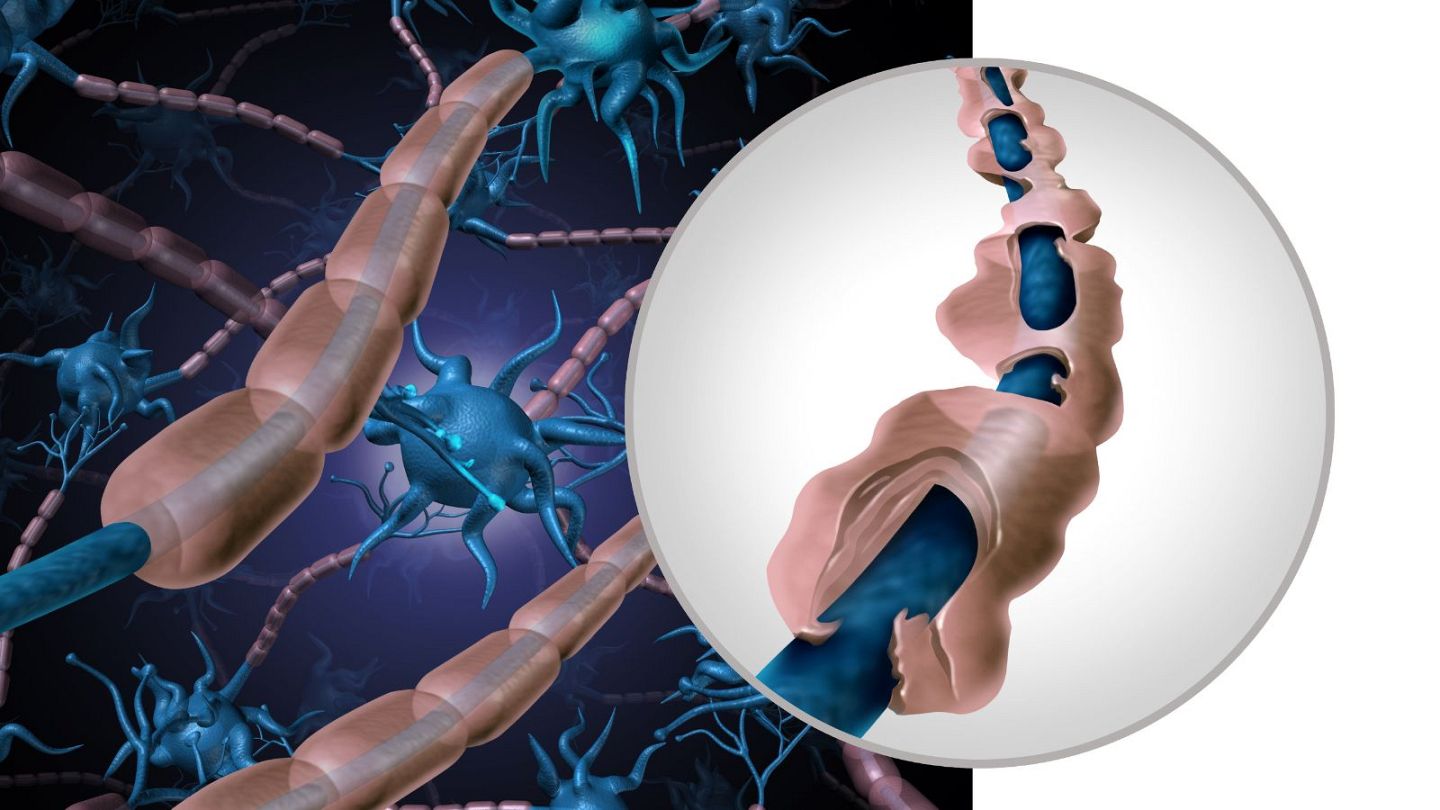
For instance, macrophages are big cells that consume pathogens; but, in MS patients, a specific kind of macrophage known as microglial cells targets nerve cells, inciting inflammation.
In MS patients, there is also damage to the myelin sheath, an insulating covering surrounding certain neurons that prevents messages from correctly traveling throughout the brain and the body.
Resetting the immune system and readjusting the white blood cells to cease attacking the nerves is how stem cell treatment is supposed to function.
‘An encouraging step’
According to Prof. Pluchino’s research, “injected cells may be able to survive and integrate in the brain, where some of them may be able to differentiate into brain cells, while some may just stay as stem cells.”
“Injected cells turn bad microglia and bad macrophages into good ones (e.g., those able to promote tissue healing and potentially also remyelination), as well as killing inflammatory white blood cells that contribute to the inflammation associated with chronic MS.”
Though it is still up for dispute, it is believed that future research may be able to repair and replace damaged nerves, which might see MS sufferers really recover and improve.
The next phase two studies will clarify if recovery from multiple sclerosis (MS) is possible and how stem cells stabilize the nervous system’s degradation.
Prof. Pluchino said, “Our findings are a step towards developing a cell therapy for treating MS. We desperately need to develop new treatments for secondary progressive MS.”
“We can move on to the next phase of clinical trials because our treatment was safe and had effects that persisted for the full 12-month trial period.”
It has taken about thirty years to get from the discovery of brain stem cells to this experimental therapeutic intervention, according to Prof. Angelo Vescovi, the co-leader of the study at the University of Milano-Bicocca.
“This work will contribute to the growing interest in this area and open the door for future, larger efficacy studies.”
“These results show that people with secondary progressive MS were safe and well-tolerated by special stem cells injected into the brain,” says Caitlin Astbury, research communications manager at the MS Society.
“This was a very tiny, early-stage research, and further clinical studies are necessary to determine if this medication is effective in treating the illness. However, this is a positive start in the direction of a different approach to treating certain MS patients.
The work appears in the Cell Stem Cells journal.





