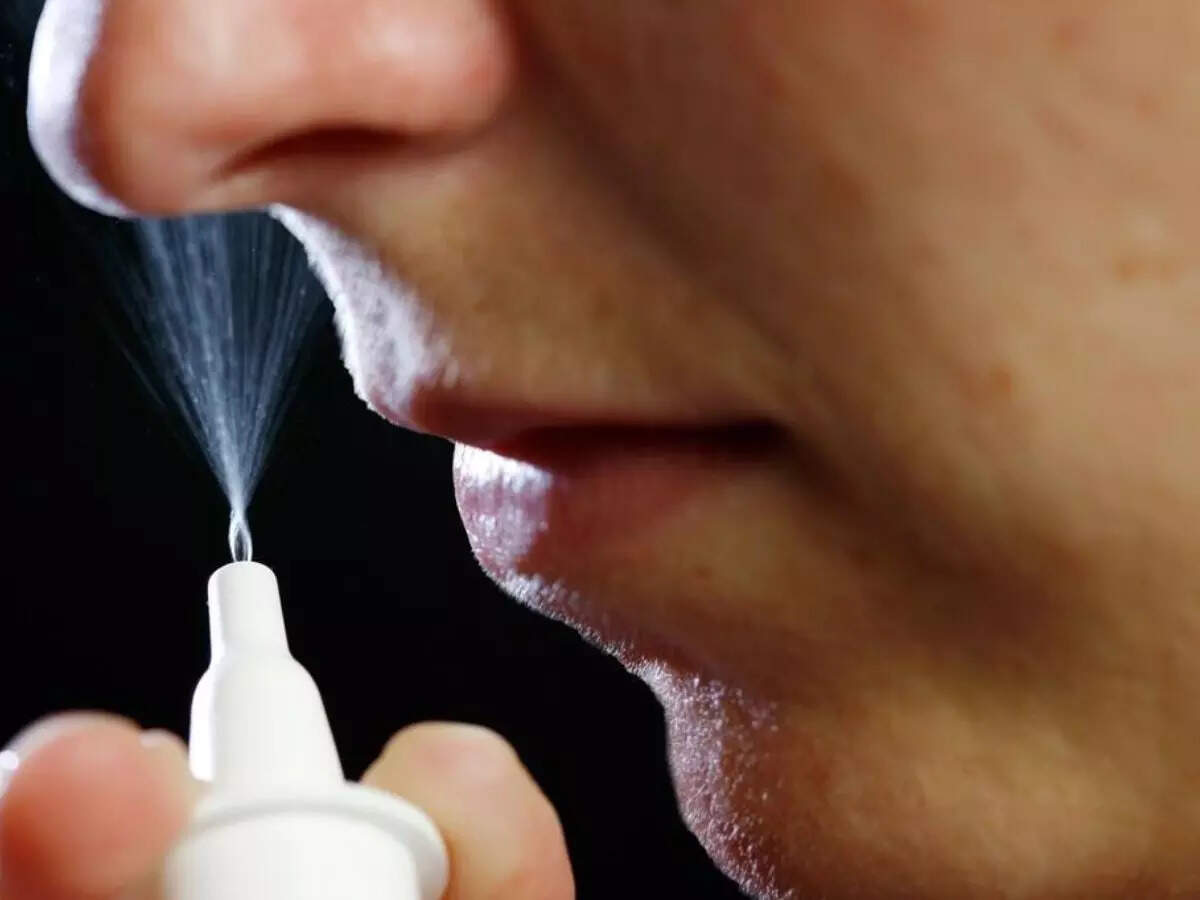
The spray is viewed as an alternative to EpiPen and other autoinjectors; it will be marketed under the Neffy brand.

As the first needle-free emergency therapy for potentially deadly allergic reactions, the Food and Drug Administration authorized ARS Pharmaceuticals’ nasal spray on Friday.
The spray, which will go by the name Neffy, is intended to serve as a substitute for the EpiPen and other autoinjectors that contain epinephrine, which is a life-saving medication used by those who are allergic to certain foods or substances. One such autoinjector is Kaleo’s Auvi-Q.
An allergic response that is severe enough to be fatal, anaphylaxis usually affects many body areas and is a medical emergency.
Neffy is a nasal spray that is intended to be used once in the nostril and is licensed for use in patients who weigh at least 66 pounds, both adult and pediatric.
Kelly Stone, an associate director of the FDA’s Center for Drug Evaluation and Research, stated that “some people, particularly children, may delay or avoid treatment due to fear of injections.” She also added that the nasal spray’s availability may lower obstacles to prompt treatment.
The approval of Neffy is predicated on four studies that examined the blood levels of adrenaline after administering Neffy or other authorized epinephrine injectable products to 175 healthy persons who did not experience anaphylaxis.
In defiance of the advice of its independent experts, the FDA last year refused to approve the spray and asked for more testing instead.